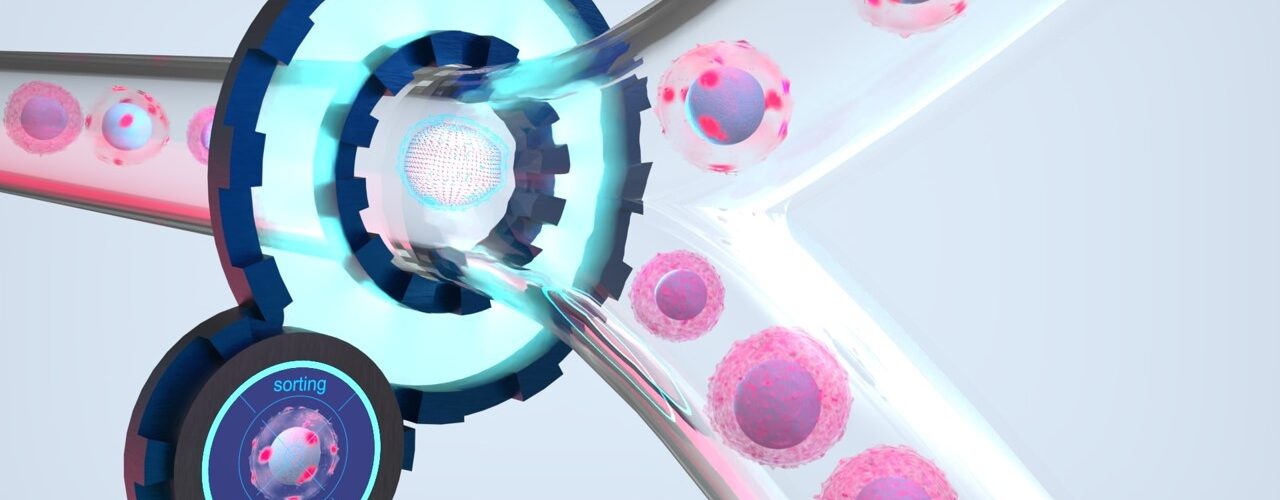
Cell sorting is an established and powerful technology in molecular biological research to distinguish and isolate specific cell types from a heterogeneous cell population. Isolation of the specific cell populations enables downstream multiomics analyses.
Fluorescence Activated Cell Sorting (FACS)
There are various methods of cell sorting with different advantages and disadvantages. One of the most commonly used methods is fluorescence activated cell sorting (FACS). In this process, cells are passed through a flow cytometer in which they are excited by lasers. According to the light scatter, size, shape, and structure can be distinguished. In addition, fluorescence-based markers are used to sort the cell population according to whether specific proteins are expressed in the cell or not.
Molecular biology research is mostly more complex than a simple 1 or 0 decision, e.g., whether a protein is expressed or not. Many biological processes in the cell depend on whether a protein is localized in a particular cell compartment or whether it is co-localized with another protein.
A good example of this is the distinction between activated and non-activated T cells. In non-activated T cells, the T cell receptor is evenly distributed across the cell membrane. When the T cell is activated, there is a redistribution of the receptors in the membrane to form clusters, which initiates a reaction cascade in the cell. Since the concentration of T cell receptors on the cell surface remains the same in both cases, a classical FACS cannot distinguish the two cell populations due to the unchanged fluorescence signal.
Image Activated Cell Sorting (IACS)
If, on the other hand, the cell can already be analyzed more precisely in the sorting process and intracellular distribution of proteins can also be taken into account, new possibilities open up for more specific differentiation of cell populations. By combining gentle microfluidics, high-resolution microscopy, and intelligent image analysis, each individual cell can be analyzed and a more accurate sorting decision can also be made based on the intracellular distribution of the fluorescence signal. For example, distribution patterns of the receptors on the activated T cells can also be detected and thus activated T cells can be distinguished from non-activated ones.
However, how does the sorting system know according to which specific intracellular localizations the sorting decision should be made?
Train-by-Example replaces tedious gating
An AI-based method is used for this purpose, which can be adapted to specific use cases by the user. It is based on a concept called “Few Shot Learning” — the AI learns what to do on the basis of a few “shots” (fewer examples). The user first feeds a small sample through the sorting system — with sorting deactivated. As the cells flow one by one through the microfluidic channels, images are taken of each cell. An image gallery of the cells builds up. Here, the user selects a few typical target cells as examples, and the AI “learns” what to sort quickly and easily based on this data. Since the AI has already been “pre-trained” with millions of fluorescent cell images, the selection of just a few target cells is sufficient to adjust the AI accordingly. The final adjustment of the AI to the specific target cells of the respective sample is fast and straightforward. Instead of tediously specifying cell attributes, the user simply clicks on the images of desired cells as examples. After customizing the AI model, the sorting process can be started and the use-case specific AI is used in real-time to sort the cell sample in the system.
IMAGO-Project for image-activated cell sorting
To use this method for image-based cell sorting, researchers from three Fraunhofer institutes have joined forces with the Charité in Berlin. This brings together expertise in microfluidics, optics, and image analysis with medical excellence to incorporate the complexity of biological processes into sorting decisions. In addition to the co-localization and distribution of proteins, one could, for example, also include the relationship between cytoplasm and nucleus, the morphology of cells and cell organelles, extracellular vesicles, or cell aggregations in the sorting decision. In this way, you can recognize the right one even among thousands of cells!
All the facts at a glance
- High-resolution image data with high imaging quality (60x magnification)
- Multispectral imaging with 3 color channels for fluorescent markers
- AI based cell classification
- “Train by example” using example images of target cells instead of time-consuming gating
- High yield, high purity
- Low shear forces, high biocompatibility due to microfluidic cell processing
- Aerosol-free cell deposition
You can find more information on our homepage: https://www.cellsorting.fraunhofer.de/
Image copyright: © Fraunhofer IIS & Fraunhofer IZI-BB




Add comment