2022 and 2023 have been breakthrough years for AI in digital pathology. Many new startups, backed by lots of venture capital, as well as established vendors have released AIs cleared for clinical routine. As of today (January 2024), a low two-digit number of digital pathology AIs with an CE-IVDR label is available. The situation in the U.S. looks a bit dimmer: So far, according to www.fda.gov, only two histopathology AIs (both by NYC based Paige.AI) have received FDA approval — compared to a couple of hundred in radiology.
Over 70 products from over 30 vendors
Our curated list of commercial digital pathology AIs currently contains over 70 products from over 30 vendors.
Vendors include Ibex, Paige.AI, Visiopharm, Indica Labs, Aiforia, Mindpeak, aetherAI, Proscia, Owkin, Visiopharm, Fraunhofer IIS/MIKAIA, and many more.
The entire list can be downloaded at the end of this blog article.
We have included all (to our best knowledge — please get in touch, if you are missing someone) digital pathology AIs with CE-IVDR or FDA clearance as well as many vendors who offer histopathology AIs for Research Use Only (RUO). For brevity, we have in some cases combined multiple AIs from the same vendor that target the same entity into a single row, e.g., Ki67/ER/PR for breast cancer.

So what entities and use cases do these AIs address? Unsurprisingly, a few mainstream use cases lead the list:
- Gleason grading for prostate cancer
- IHC scoring for breast cancer: Ki67, ER, PR, HER2, HER2-low
- PD-L1 scoring
- (Micro-) metastasis detection for lymph nodes
Why were these AIs published first?
The broad field of histopathology could use hundreds of AIs (see further below why). Yet, the list of commercial AIs contains quite a few duplicates. Why did so many companies’ strategists arrive at the same conclusion as to which AIs the pathology world needs first?
I can only make assumptions, but likely they looked at the following trifactor:
- Incidence
How frequently could pathologists make use of a particular AI? From a commercial perspective it helps addressing use cases first that are well known and frequently carried out by many pathologists. Tumors with high incidence are breast, prostate, lung, etc. - Regulatory
Start with low-risk AIs first that count cells or direct the pathologist’s gaze to where a tumor might be. Why start with more difficult use cases like filtering uneventful slides out, claiming “You do not even have to look at this tissue. It does not contain any pathologies”? For the time being, pathologists will have to double-check the AI’s prediction and sign off on it, and that’s probably a good idea.
The question is not “AI or pathologist?“, but rather “AI-assisted pathologist or oldschool AI-sceptical pathologist?” - Acceptance
One step at a time.
Adoption of AI in digital pathology is a major revolution anyway, following in the footsteps of the introductions of (1) immunohistochemistry, (2) molecular pathology, and (3) digital pathology.
Most early commercial AIs mimic a well known score (like tumor grading, IHC scoring) that is already assessed manually today and therefore well-understood by all pathologists. Study designs are straight forward and pathologists can compare the AIs’ prediction to their own in order to build trust in these new smart assistants.
However, a lot of potential also lies in AIs that are limited in the sense that they merely automate one of today’s scores (which are designed so that a pathologist can manually execute them within a few minutes). Instead, an AI’s full power can be unleashed by using it to predict an endpoint (e.g., high-risk vs. low-risk; responder vs. non-responder) instead of just predicting a proxy. Let’s call this class Digital Pathology AI 2.0. Already, first examples of this class are showing up, for instance StratiPath Breast or Histotype Px® Colorectal.
How many AIs do we need?
Keep in mind that clinical AIs are only cleared for a very narrow use case. An automated PD-L1 scoring AI, for example, may only be cleared for one particular entity, such as non-small-cell lung cancer (NSCLC) and one particular PD-L1 clone by one assay vendor.
As a consequence, hundreds of AIs are required for the broad spectrum of …
- Entities (breast, prostate, colon, brain, …)
- Stains (H&E, various IHC, …)
- Scores (finding tumor, grading tumor, assessing cell ratios, assessing expression level, …)
… that pathologists cover (including their sub-disciplines dermato-, neuro-, and nephropathology).
Digital Pathology AI in the workflow
How are Digital Pathology AIs integrated into the lab workflow?
Pathologists certainly do not want to — and should not have to — open a new software for every single AI they are utilizing. Not only would this be very annoying, but having to switch between multiple whole-slide viewers or softwares in general is also prone to errors. In fact, in clinical routine, pathologists should not have to launch any AIs at all. Ideally, a chain of AIs is automatically kicked off as soon as a new scan becomes available.
The workflow looks as follows:
- Load Scanner
Assistant loads new glass slides into scanner. - Scan
Scanner digitizes glass slides and produces so called whole-slide-image (WSI), and stores it on a local folder. - Upload & Ingest
A watchdog software called “bridge” immediately detects the new scan, uploads it into the cloud, and ingests it into the Image Management System (IMS), sometimes also called Histo-PACS. - AI Analysis
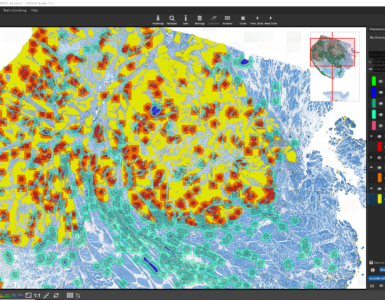
It does not make sense to run all AIs on each scan. A Ki67 AI would (hopefully) not find any DAB+ nuclei in a H&E stained lymph node section. Such an analysis would be nonesense and a waste of time and ressources. Now, do we need an initial Tissue & Stain Identification AI only to figure out which diagnostic AIs to launch? No! Instead, the tissue and stain type are coded into the barcode or QR-code sticker on the glass slide. Depending on this information, only the relevant AIs are automatically launched. Today’s AIs in some cases need up to 30 minutes per scan. The result is reported back to the IMS. - Open and execute case
When the pathologist later opens the case, all the AI magic has already happened. The IMS can sort the case-list by tumor-probability. When the pathologists opens a scan, overlays such as tumor-probability heatmaps or PD-L1 hotspots are available, including quantitative ROI- or slide-level metrics.
Only steps (1) and (5) are manual.
Steps (2) to (4) are automated.
In order for this scenario to work, AI vendors need to plug-in their AIs into the various IMS systems. Or to put it the other way around, IMS vendors need to open their platforms for AIs from third parties. We will publish a list of digital pathology ready IMS systems soon on this blog.
Free download of curated list of Digital Pathology AIs
You can download the full curated list of commercial Digital Pathology AIs from here for free:
Last updated 02.05.2024
Copyright: kajhinkson/unsplash





Add comment